Get support how and when you need it
It's easy to set up a rapid antigen testing program with the BD Veritor™ Plus System. Get access to a pre-recorded training session, an on-demand e-Learning platform, quick reference guides, and training videos, or book a 1:1 training with a bilingual trainer on the BD Veritor™ Plus System Training Site.
Simple, reliable COVID-19 test results in 15 minutes
-
Simple workflow utilizing a nasal swab sample
-
Includes prefilled, unitized reagent tubes to reduce the hands-on time
- Streamline registration by pre-loading a list of test subjects or scanning test subject's identification to pre-populate information
- Share test results with test subjects through a secure email system
- Securely store test results and prepare reports automatically without the need for paper records
Learning Objectives:
- Defining value from the patient perspective
- Examining global readiness and enablers of value-based healthcare and procurement goals
- Understanding the patient perspective in value-based healthcare by showcasing patient-reported experience and outcome measurement
- Showcasing value realized to date via a case study about a Patient Reported Experience Measure that informed and validated value-based healthcare approaches
- Operationalizing value-based procurement in health systems to enable value-based healthcare
Lets connect
Securely store and send results with the ImageMover App
-
Streamline registration by pre-loading a list of test subjects or scanning test subject's identification to pre-populate information
-
Share test results with test subjects through a secure email system
-
Securely store test results and prepare reports automatically without the need for paper records
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.

Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
Pellentesque habitant morbi
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
Pellentesque habitant morbi
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
- Tristique senectus et netus et malesuada
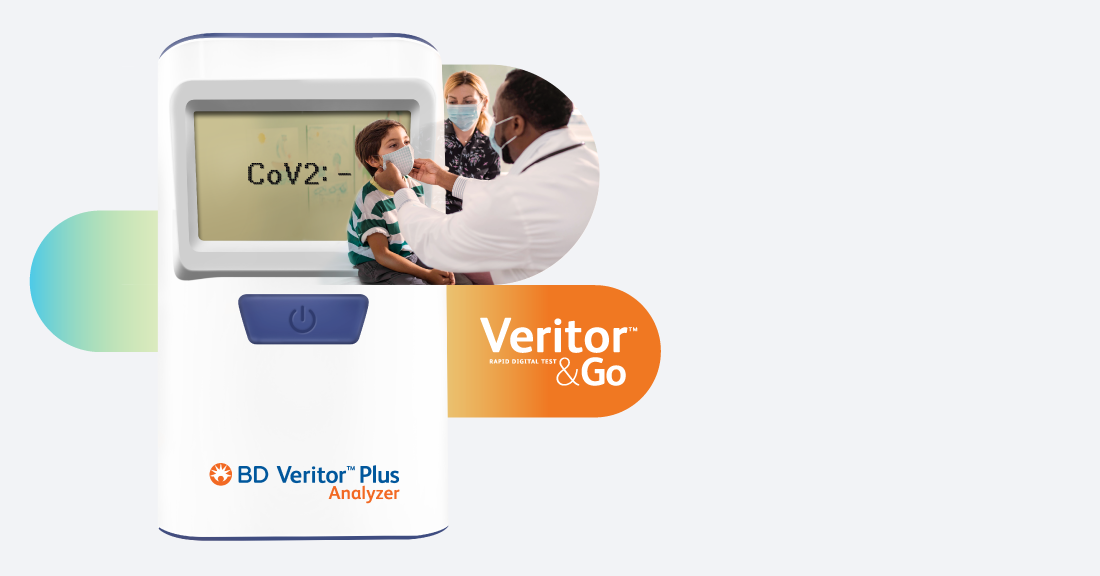
For unambiguous results you can trust, the choice is clear
The BD Veritor™ Plus System is an easy-to-use, one-button device that offers reliable results through a digitally read test assay that leaves little room for misinterpretation. The instrument analyzes and corrects for nonspecific binding and detects positives not recognized by the unaided eye to provide an objective results that you can count on.
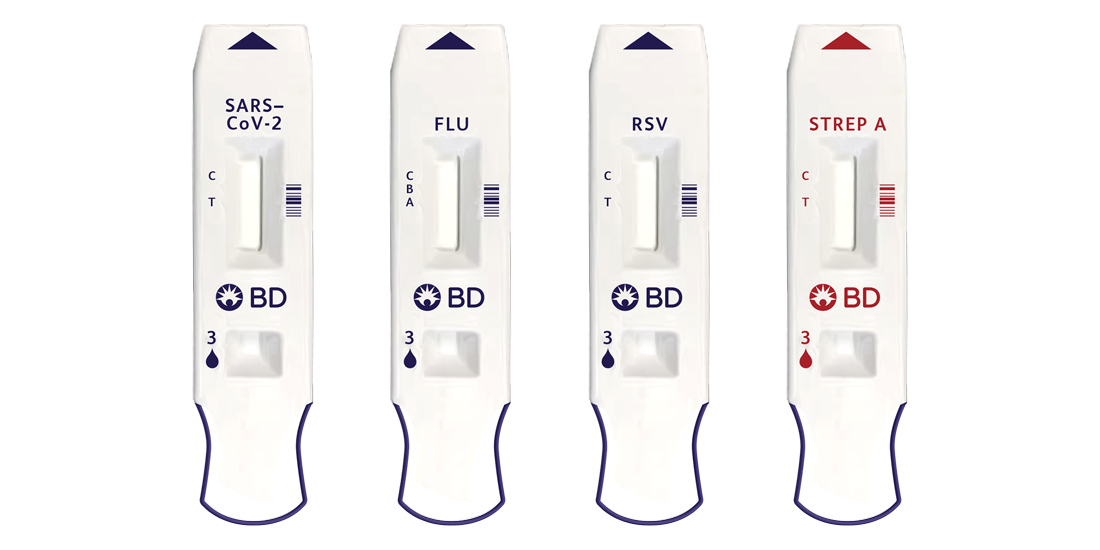
The BD Veritor™ Plus System offers convenient point-of-care testing for:
Store Tab


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized
Store Tab


360° rotation knob is effectively sized, contoured and located to enable easy instrument rotation.

360° rotation knob is effectively sized

360° rotation knob is effectively sized

360° rotation knob is effectively sized

Hospitals and healthcare facilities
BD is a market leader in hospital products that can reduce the incidence of sharps injuries and exposure to bloodborne pathogens. Patient safety has been a focus of BD innovation for years, not only in the United States but also around the world. Working closely with organizations like the International Safety Center (EPINet) is an important part of our efforts to keep patients and workers safe.
Safety syringes and needles >>
Hazardous drug safety >>
Infection prevention >>
More Resources:
Watch our recent webinar: On-site COVID-19 testing for workplaces here
Quick Reference Guides:
- Workflow Overview
- Analyze Now Guide
- Walk Away Mode Guide
- Workflow - without the use of the BD Veritor™ Plus Analyzer
For more information, please view the following videos on:
ImageMover Videos:
-
Workflow Overview
-
Single Test Workflow
-
Batch Testing Workflow
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Pellentesque habitant morbi tristique senectus et netus et malesuada fames ac turpis egestas vestibulum tortor quam.
Let's have a conversation
- This test has been authorized for sale in Canada by Health Canada under Interim Order
- This test is only authorized for the duration of the Interim Order Respecting the Importation and Sale of Medical Devices for Use in Relation to COVID-19, unless the authorization is terminated or revoked sooner
- This test has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
BD is committed to keeping your personal data protected and secure. More information on how we protect your personal data can be found in our privacy statement and our cookie policy.

